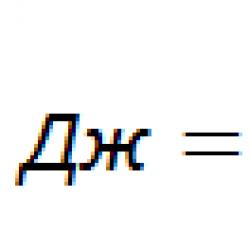Salicylic acid derivatives as drugs. Salicylic acid and its salts, esters and amides as drugs. Salicylic acid derivatives
Salicylic acid (from the Latin Salix - willow, from the bark of which it was first isolated) - 2-hydroxybenzoic acid, C 6 H 4 (OH) COOH; colorless crystals, highly soluble in ethanol, diethyl ether and other polar organic solvents, poorly soluble in water (1.8 g/l at 20 °C).
Isolated from willow bark by the Italian chemist Rafael Piria and then synthesized by him.
Occurs in nature in plants in the form of derivatives - mainly in the form of a methyl ester glycoside (in particular, salicylic acid was first isolated from the bark of willow (Salix L.), hence the name), free salicylic acid along with salicylic aldehyde in small quantities found in essential oil extracted from the flowers of some species of spirea (Spiraea ulmaria, Spiraea digitata).
Physical properties
Salicylic acid is easily soluble in ethanol and diethyl ether, and slightly soluble in carbon disulfide.
Physiological role and action of salicylates.
Effect on humans and animals:
Salicylic acid and salicylates, as well as its esters (methyl salicylate) and other synthetic derivatives of salicylic acid (for example, acetylsalicylic acid - aspirin), have a pronounced anti-inflammatory effect.
Application in medicine:
Salicylic acid is the active component of willow bark. Back in the 19th century. it was used to treat rheumatism and uric acid diathesis, and today this substance is synthesized in large quantities, as it serves as the basis for the production of many drugs.
Salicylic acid has weak antiseptic, irritant and keratolytic (in high concentrations) properties and is used externally in medicine in ointments, pastes, powders and solutions in the treatment of skin diseases; is part of Lassara paste, galmanin powder, corn liquid and corn plaster preparations.
Salicylic acid derivatives are also used in medicine (sodium salicylate), its amide (salicylamide) and acetylsalicylic acid (aspirin) are used as antipyretic, antirheumatic, anti-inflammatory and analgesic agents; phenyl salicylate - as an antiseptic, para-aminosalicylic acid (structurally similar to para-aminobenzoic acid, necessary for tuberculous mycobacteria, and therefore metabolically competing with it) - as a specific anti-tuberculosis agent.
Other Applications
Due to its antiseptic effect, salicylic acid is used in food preservation; It is also used in the production of azo dyes, aromatic substances (salicylic acid esters), for the colorimetric determination of Fe and Cu, and for separating thorium from other elements.
Salicylic acid, its acidic properties.
Salicylic (o-hydroxybenzoic) acid is a phenolic acid. As a compound with ortho functional groups, it is easily decarboxylated when heated to form phenol. Salicylic acid is soluble in water and is a stronger acid than benzoic acid (pKa = 4.17). The increased stability of the salicylate ion is explained by the formation of an intramolecular hydrogen bond.
Salicylic acid gives intense coloration with iron (III) chloride, which is due to the presence of a free phenolic hydroxyl group.
It has antirheumatic, antipyretic and antifungal effects, but since it is a strong acid, it is used only externally. Its derivatives - salts or esters - are used internally.
The following salicylic acid derivatives find practical application:

Sodium salicylate has anti-inflammatory, antipyretic and analgesic effects.

Due to its irritating and toxic effects, methyl salicylate is used only externally; included in ointments and rubs.

Phenyl salicylate is not hydrolyzed in the acidic environment of the stomach, but decomposes only in the intestine. It is used as a disinfectant for intestinal diseases, and is also used as a material for the protective coatings of certain medications that are unstable in the acidic environment of the stomach.

Acetylsalicylic acid (aspirin) has anti-inflammatory, antipyretic and analgesic effects, and is also used as an antiplatelet agent (prevents platelet aggregation and thrombus formation).
Salicylates have an anti-inflammatory effect.
Salicylic acid- Acidum salicylicum.
White small needle-shaped crystals or light crystalline powder, odorless, slightly bitter taste, soluble in 500 parts of cold and 15 parts of boiling water.
Topically, salicylic acid slowly but strongly irritates sensory nerve receptors.
Salicylic acid is prescribed internally for infectious diseases of the digestive tract. Particularly favorable results were observed with infectious diarrhea in calves.
Sodium salicylate- Natrii salicylas, C 7 H 5 Na0 h. White crystalline powder or small flakes, odorless, sweetish-salty taste; dissolve in 1 part water and 6 parts alcohol.
Sodium salicylate has a bactericidal effect, similar to sulfonamide drugs in the treatment of wounds. It has a bacteriostatic effect against pathogenic anaerobes, streptococci and staphylococci. Apply powder or 25% aqueous salt solution to wounds.
Sodium salicylate has a pronounced antihemoagglutinating effect and is used to treat hemolytic shock in animals (intravenous 10% solution).
Acetylsalicylic acid- Acidum acetylsalicylicum. Salicylic ester of acetic acid, White needle-shaped crystals or plates of slightly acidic taste, soluble in 300 parts of water and 20 parts of alcohol, easily soluble in alkaline solutions. Aqueous and alcoholic solutions of acid reaction.
Acetylsalicylic acid is a good antipyretic, antirheumatic and analgesic agent.
The pharmacological effect occurs only in certain disorders of the body:
antipyretic - for fever,
analgesic - for neuralgia,
anti-inflammatory - with excessive hyaluronidase activity.
ALCOHOL
The names of alcohol (alcohol) preparations are determined by radicals that are associated with hydroxyl (ethyl - ethyl, methyl - methyl, etc.);
Ethanol(wine alcohol, ethanol) - Spiritus aethylicus, C 2 H 5 OH.
Ethyl alcohol is a colorless, transparent, highly flammable volatile liquid with a peculiar odor and pungent taste. Easily mixes with water, removes water from living cells and from chemical solutions.
There are 95, 90, 70 and 40% ethyl alcohol. If the alcohol concentration is not indicated, use 95% alcohol.
The action is local. When applying alcohol to the skin or mucous membranes, a cold sensation is first felt, and then a burning sensation and hyperemia appears.
When administered subcutaneously alcohol at a concentration of 40% at the injection site causes severe irritation and even tissue inflammation, lasting several days. At 50-60% concentration it can cause tissue necrosis.
Bactericidal effect alcohol is very unique: absolute alcohol does not kill microbes, and 50-70.% alcohol has the highest bactericidal effect.
Suction. Alcohol is absorbed by the mucous membranes very quickly: within 5-10 minutes symptoms of the effect on the central nervous system appear, and after 15-25 minutes the effect is often fully manifested.
The action is resorptive. In large doses, alcohol acts similar to drugs. In the stage of complete anesthesia, the respiratory center is sharply depressed, and breathing becomes shallow. A slight overdose in these cases ends in the death of the animal. The narcotic state lasts from 40 minutes to 3 hours and is replaced by long sleep.
Breathing during light anesthesia weakens slightly, and with small doses it often even increases.
Alcohol is not very toxic for the heart. In an animal under the influence of alcohol, heart contractions become more frequent and often intensify. This is largely due to its reflex effect, and partly due to the expansion of the coronary vessels. Blood pressure initially (due to a reflex effect) increases and then gradually decreases as a result of weakening vasoconstrictor impulses, especially in peripheral vessels.
Metabolism. Alcohol is an energy source; when it oxidizes, it releases a significant amount of heat. The energy obtained as a result of the combustion of alcohol activates the functional activity of organs.
Application. As a narcotic drug, alcohol is prescribed orally and intravenously.
As an analgesic, antifermentative and antiseptic, it is prescribed for acute dilatation of the stomach, severe fermentation processes, convulsive colic and intestinal atony, as well as an analgesic for inflammation of the brain, convulsive cough, and canine distemper.
As a stimulant and energizing agent, alcohol is prescribed after hard work, for general weakness, collapse, for infectious diseases, after severe blood loss, for poisoning with inhaled drugs, prolonged labor, as well as for septic fever, exhaustion and progressive weakness.
For disinfection of the surgical field, surgeon's hands, instruments, dressings.
The use of alcohol in large doses is contraindicated in all forms of sharp weakening of the physiological function of the connective tissue system, with organic heart defects and kidney diseases.
Related information.
Currently, salicylic acid derivatives are widely used in medicine: sodium salicylate, salicylamide, acetyl salicylic acid. Salicylates are used for various diseases. They have antipyretic, anti-inflammatory and analgesic effects.
Salicylic acid is used as an external antiseptic and antifungal agent. The drug is not used internally, as it has an irritating effect. In its pure form, it is used only to soften calluses and to accelerate the breakthrough of pus in the case of treating abscesses.
Salicylic acid was obtained from salicin, which in turn was isolated from willow bark. Salicylic acid is also found in other plants: poplar buds, jasmine, olives, even oranges, cherries and plums.
Salicylic acid methyl ester is not ingested due to its strong irritating effect. It is included in all kinds of rubbing: “sanitas”, “naftalgin”, etc.
Sodium salicylate is used orally for rheumatism, arthritis, and neuralgia. Salicylamide - salicylic acid amide is prescribed for neuralgia, migraine, rheumatism and inflammatory diseases of the upper respiratory tract.
All drugs that have properties similar to salicylates are classified into a special group of “non-narcotic analgesics (painkillers).” These drugs include acetylsalicylic acid, salicylamide, amidopyrine, analgin, butadione, phenacetin, paracetamol, indomethacin. They should be distinguished from another group of analgesics (morphine, heroin, promedol and others), which can cause drug addiction.
Acetylsalicylic acid analysis
Preliminary information
Aspirin has been used for 80 years and people still haven’t learned how to take it correctly. In the 1980s, the brand name of aspirin was replaced by a name derived from the chemical structure of the substance. Aspirin became known as acetylsalicylic acid.
Acetylsalicylic acid is sold in pharmacies as a remedy for headaches.
Acetylsalicylic acid is also used as an anti-inflammatory agent. It is especially effective for inflammations that occur with edema (pleurisy, sore throat, flu, pneumonia, influenza).
The use of acetylsalicylic acid prevents its side effects. The most common symptoms are stomach irritation (nausea and vomiting). Ringing in the ears and hearing loss are less common. Bleeding in the gastrointestinal tract has been reported. Stomach damage occurs easily if the acidity of gastric juice is increased, there is gastritis or a stomach ulcer.
The irritating effect of the drug can be completely prevented if the tablets are taken correctly. First of all, it is strictly not recommended to swallow them whole, but should first be dissolved in water. Before taking acetylsalicylic acid, you should drink 1 / 2 - 1 glass of alkaline drink (mineral water, soda solution), dissolve the tablet in a spoon of water And swallow it, then eat jelly or broth. It is not recommended to take the drug during or after meals. It should be taken 20-30 minutes before meals.
Equipment and reagents: 5 ml pipettes (3 pcs.), medical pipettes (2 pcs.), test tube (1 pc.), test tube rack (1 pc.), 25 ml conical flask (1 pc.), titration burette (1 pc. pcs.), clamp for test tubes (1 pc.), spatula (1 pc.), alcohol lamp or gas burner (1 pc.), scales, acetylsalicylic acid, potassium hydroxide solution (0.5 M), sulfuric acid solution (1 pc. :1), ethanol, distilled water, phenolphthalein, sodium hydroxide solution (0.1 M).
Progress
I . QUALITATIVE ANALYSIS
1. Place acetylsalicylic acid (on the tip of a spatula) into a test tube.
2. Dissolve in 5 ml of 0.5 M potassium hydroxide solution.
3. Gently boil the test tube for approximately 3 minutes.
4. After cooling, acidify the solution with sulfuric acid (1:1). A white crystalline precipitate forms and the smell of acetic acid is felt.
RESULTS:
5.2. Hydrolysis of acetylsalicylic acid (aspirin)
Preliminary information
An important characteristic of medicinal products is purity (the absence of impurities or decomposition products). If the shelf life of the drug exceeds the period indicated on its packaging, then this medicine cannot be used. It may contain impurities harmful to the body. Do not use medications that are stored incorrectly. As an example of a drug that is not resistant to moisture and heat, acetylsalicylic acid can be analyzed.
Equipment and reagents: aspirin tablet, ethanol, test tube rack (1 pc.), test tubes (3 pcs.), 2 ml pipettes (2 pcs.), pipettes (medical) (2 pcs.), 50 ml conical flask (1 pc. ), 0.1 M alcohol solution of salicylic acid, 10% aqueous solution of iron (III) chloride, sulfuric acid solution (1:1), glass rod, tweezers, 25 ml measuring cylinder (2 pcs.), laboratory stand (1 pc.), clamp for test tubes (12 pcs.), alcohol lamp (or test tube heater) (1 pc.).
Progress
I. TESTING ASPIRIN FOR THE PRESENCE OF SALICYLIC ACID
1. Place a grain of aspirin in a test tube.
2. Dissolve aspirin by shaking in 5 drops of water.
3. Add a drop of iron (III) chloride solution. Observe whether a purple color appears.
II. PARTIAL HYDROLYSIS OF ASPIRIN
1. Place half a teaspoon of aspirin in the flask.
2. Dissolve aspirin in 20 ml of water.
3. Heat the mixture for 4-5 minutes.
III. QUALITATIVE ANALYSIS OF HYDROLYSIS PRODUCT (SALICYLIC ACID)
1. Pour 5 drops of the reaction mixture into a test tube.
2. Add a drop of iron(III) chloride solution. The solution acquires a lilac color, which proves partial hydrolysis of aspirin.
IV. COMPLETE HYDROLYSIS OF ASPIRIN
1. Add 1 ml of a solution (1:1) of sulfuric acid to the partially hydrolyzed aspirin solution.
2. Heat the mixture for 8-10 minutes.
When the solution cools, white needle-shaped crystals are released.
RESULTS:
Novocaine analysis
Preliminary information
Local anesthetic drugs are obtained based on the aniline derivative - para-aminobenzoic acid. These include esters of p-aminobenzoic acid - anesthesin, novocaine, dicaine.
Novocaine is an odorless white powder with a bitter taste and causes a numb feeling on the tongue. The drug is easily soluble in water.
Novocaine is one of the most widely used local anesthetics. Considering the short-term effect of novocaine, it is often prescribed together with adrenaline, which, due to its vasoconstrictive effect, slows down the absorption of novocaine and extends its duration of action. The highest single oral dose is 0.25 g, the highest daily dose is 0.75 g.
Store in well-sealed dark glass jars.
Equipment and reagents: test tubes (2 pcs.), 1 ml pipette (1 pc.), pipettes (medical) (3 pcs.), spatula (1 pc.), test tube rack, novocaine, nitric acid solution (2 M), nitrate solution silver (1%), ammonium hydroxide solution (6%).
Progress
QUALITATIVE ANALYSIS (WITH SILVER NITRATE)
1. Place the drug on the tip of a spatula into the test tube.
2. Dissolve the contents of the test tube in 1 ml of water.
3. Add 5 drops of 2 M nitric acid solution.
4. Add 5 drops of silver nitrate solution. A white precipitate forms.
5. Pour some of the contents of the test tube into another test tube.
6. Add ammonium hydroxide solution.
Security measures
Do not pour silver nitrate and nitric acid solutions down the sink. Drain them only into special drains.
RESULTS:
Amidopyrine analysis
Preliminary information
Among analgesics (painkillers), there is a large group of non-narcotic substances that have anti-inflammatory and antipyretic properties. These include not only aspirin, but amidopyrine, antipyrine, analgin and butadiene. All of them are obtained synthetically. Amidopyrine (pyramidon) is a white crystalline powder with a slightly bitter taste, odorless, and poorly soluble in water. Obtained from antipyrine. Amidopyrine is part of a large number of medicinal substances and is used as an antipyretic and analgesic. And anti-inflammatory agent for headaches, neuralgia, arthritis and articular rheumatism. It can be prescribed orally in combination with caffeine, phenacetin, sodium barbital, etc. Codeine preparations are often used in combination with amidopyrine (pentalgin, cofadine) for headaches, neuralgia, etc. With long-term use, amidopyrine produces a number of undesirable side effects: changes in the blood count and etc. Included in “piraminal”, “pentalgin”, etc. Available in powder and tablets of 0.25 g.
The highest single oral dose is 0.5 g, the highest daily dose is 1.5 g.
Equipment and reagents: test tube (1 pc.), test tube rack, spatula (1 pc.), 2 ml pipette (1 pc.), 1 ml pipette (1 pc.), pipette (medical) (1 pc.), spatula ( 1 piece), amidopyrine, iron (III) chloride solution (0.5 M), hydrochloric acid solution (1:3), water.
Progress
3. Add 1 drop of iron (III) chloride solution. A blue color that quickly disappears is formed, followed by a brown flocculent precipitate.
4. Add 1 ml of hydrochloric acid solution. A blue-violet color is formed.
Security measures
Do not pour hydrochloric acid and amidopyrine solution down the sink. Drain them only into special drains.
RESULTS:
Analgin analysis
Preliminary information
Analgin is an odorless white powder with a bitter taste, easily soluble in water. Aqueous solutions turn yellow when standing. Analgin has an analgesic, antipyretic and anti-inflammatory effect. Analgin is most widely used as a painkiller. In febrile conditions, analgin has a rapid antipyretic effect. Compared to other antipyretics, it gives a much more reliable and lasting effect. Analgin is used as an antirheumatic drug for acute articular and muscular rheumatism.
Being a derivative of antipyrine, analgin is superior in intensity and speed of action to antipyrine and its derivative amidopyrine. Good solubility and absorption determine the rapid action of analgin when taken orally. Available in powder and tablets of 0.25 g and 0.5 g, as well as in ampoules. The highest single dose is 1 g, the highest daily dose is 3 g. Doses are determined by the doctor depending on the disease. Store analgin in a place protected from light in well-sealed dark-colored jars.
Equipment and reagents: test tube (1 pc.), test tube rack, spatula (1 pc.), 2 ml pipette (1 pc.), pipettes (medical) (2 pcs.), analgin, iron (III) chloride solution (0.5 M), hydrochloric acid solution (1:3), water.
Progress
QUALITATIVE ANALYSIS (WITH IRON CHLORIDE)
1. Place the drug on the tip of a spatula into the test tube.
2. Dissolve the contents in 2 ml of water.
3. Add 1-2 drops of hydrochloric acid solution.
4. Add 1 drop of iron (III) chloride solution. A blue color is formed, turning into red, then the solution becomes discolored.
Security measures
Do not pour hydrochloric acid solution into the sink. Drain it only into a special drain.
RESULTS:
Caffeine Analysis
Preliminary information
The main natural source of caffeine is tea production waste. Caffeine is also found in coffee beans. Currently, cheaper synthetic and semi-synthetic methods are used to obtain caffeine. With tea and coffee, a constant flow of biologically active substances enters the human body throughout life. The most potent substance in both drinks is caffeine.
Coffee has a stronger effect, but less long-lasting than tea. In large doses, coffee, unlike tea, causes irritation of the intestinal mucosa and also contributes to heart and vascular diseases. Tea is widely used as a health remedy. Caffeine appears as white silky needle-shaped crystals with a slightly bitter taste. It erodes in the air. Caffeine is used as a central nervous system stimulant and cardiotoxic agent. The stimulating effect of caffeine leads to increased mental and physical performance, but in large doses its use leads to depletion of nerve cells. In addition, caffeine is used for drug poisoning and cerebral vascular spasms.
Available in powder. The highest single oral dose is 0.3 g, the highest daily dose is 1 g. Should be stored in a well-sealed container. Caffeine is included in drugs for relieving headaches - askofen, citramon, pirkofen, piraminal, sedalgin. The shelf life of these drugs is 4 years or more.
Equipment and reagents: test tube (1 pc.), test tube rack, caffeine solution (10%), sulfuric acid solution (1 M), iodine solution (0.1 N).
Progress
QUALITATIVE ANALYSIS
1. Pour 1 ml of caffeine solution into a test tube.
2. Add 1 drop of sulfuric acid solution.
3. Add 2 drops of iodine solution. Observe the formation of a brown precipitate.
Security measures
Do not pour sulfuric acid solution into the sink. Drain it only into a special drain.
RESULTS:
6. Paracetamol analysis
Preliminary information
Paracetamol or acetaminophen- a drug, analgesic and antipyretic from the anilides group, has an antipyretic effect. It is a widely used central non-narcotic analgesic, has rather weak anti-inflammatory properties (and therefore does not have the associated side effects characteristic of NSAIDs). At the same time, it can cause problems with the liver, circulatory system and kidneys. The risk of violations of these organs and systems increases when drinking alcohol at the same time, therefore, people who drink alcohol are recommended to use a reduced dose of paracetamol.
The mechanism of action and safety profile of paracetamol have been well studied, its effectiveness has been clinically tested, and therefore this drug is included in the list of essential medicines of the World Health Organization, as well as in the list of vital and essential medicines approved by order of the Government of the Russian Federation.
White or white with a creamy or pink tint crystalline powder. Easily soluble in alcohol, insoluble in water.
Inhibits the synthesis of PG and reduces the excitability of the thermoregulation center of the hypothalamus. Rapidly absorbed from the gastrointestinal tract and binds to plasma proteins. T1/2 from plasma 1–4 hours. Metabolized in the liver to form paracetamol glucuronide and sulfate. Excreted by the kidneys mainly in the form of conjugation products, less than 5% is excreted unchanged.
Application. Pain of mild and moderate intensity (headache and toothache, migraine, back pain, arthralgia, myalgia, neuralgia, menalgia), febrile syndrome due to colds.
Contraindications. Hypersensitivity, impaired renal and liver function, alcoholism, children (up to 6 years).
Progress
- Complexation of paracetamol with Fe 3+ cations
To 1 ml of paracetamol solution add 3-4 drops of a 10% FeCl2 solution. The reaction occurs due to the presence of phenolic hydroxyl in the paracetamol molecule, which participates in complex formation with Fe 3+ ions.
- Acid hydrolysis of paracetamol:
Add 0.5 ml to 1 ml of paracetamol solution. 2M HCl solution, heat the mixture to a boil and boil it for 1 minute. Then cool the test tube and carefully sniff its contents. There is a smell of acetic acid.
Send your good work in the knowledge base is simple. Use the form below
Students, graduate students, young scientists who use the knowledge base in their studies and work will be very grateful to you.
Posted on http://www.Allbest.ru/
Introduction
Salicylic acid group drugs are classic antirheumatic drugs. In addition to anti-inflammatory, they have a pronounced antipyretic and analgesic effect. The anti-inflammatory effect of salicylic drugs is not associated with an antimicrobial effect, but perhaps depends on their ability to stimulate the release of adrenocorticotropic hormone by the anterior pituitary gland. This hormone, in turn, enhances the secretion of hormones from the adrenal cortex, which have a powerful anti-inflammatory effect.
Salicylic acid and its derivatives
Salicylic acid(CaK) is an aromatic phenolic hydroxy acid, the hydroxyl group of which is bonded to the benzene ring.
This is a colorless crystalline substance, easily soluble in ethanol, diethyl ether and poorly soluble in cold water (1.8 g/l at 20C). Melting point is 159 C, and boiling point is 211 C (20 mm Hg)
CaK has two acidity centers - a carboxyl and a phenolic hydroxyl group and, according to its chemical properties, manifests itself as a monohydric phenol and a monobasic acid (pK = 2.98).
When CaA interacts with strong bases, salts are formed both at the carboxyl group and with the participation of a weaker acid center - the phenolic hydroxyl group (Fig. 1.2 A).
CaA displaces weak acids from their salts, such as carbonic acid.
When the carboxyl group of CaA interacts with alcohols, esters are formed. This acid is also capable of forming ethers and esters due to the phenolic hydroxyl group; when acetylated with acetic anhydride, acetylsalicylic acid is formed.
Food sources
In nature, CaA is found in the form of its methyl ester glycoside in plant essential oils. Unripe fruits and vegetables are natural sources of salicylic acid, including blackberries, blueberries, melons, dates, grapes, kiwi, guavas, apricots, green peppers, tomatoes, olives, radishes and chicory; also mushrooms. Some herbs and spices contain fairly high amounts, while meat, poultry, fish, eggs and dairy products contain little or no salicylates. Of the legumes, seeds, nuts and grains, only almonds, water chestnuts and peanuts contain them in significant quantities.
The main industrial method for the synthesis of CaA and its derivatives) is the carboxylation of dry sodium phenolate by the action of CO2 at a pressure of 0.6 MPa, a temperature of 185 C for 8-10 hours (Kolbe-Schmitt reaction) (Fig. 1).
SaK derivatives
This group may include salicylic acid esters and salicylic acid amide derivatives. Salicylic acid forms esters both with organic acids (I) due to interaction with phenolic hydroxyl, and with alcohols or phenols (II) due to interaction with the carboxyl group. Salicylic acid amide derivatives have the general formula (III): (Fig. 2)
CaA and its derivatives - sodium salicylate, salicylamide, acetylsalicylic acid (AA), lysine acetylsalicylate, salol - are important medicinal substances. SaK is an antiseptic, irritant and keratolytic agent. It is included in ointments, pastes, powders and solutions for the treatment of skin diseases and fungal nail diseases. CaA is also used as a preservative for some food products and an intermediate in the synthesis of dyes and fungicides. Sodium salicylate, salicylamide, acetylsalicylic acid, lysine acetylsalicylate are known as antipyretic, anti-inflammatory and analgesic agents; phenyl ether (phenyl salicylate, salol) - antiseptic; methysalicylate - antirheumatic agent; p-aminosalicylic acid is an anti-tuberculosis agent.
Aspirin-- acetylsalicylic acid -- C6H4(OCOCH3)COOH -- is widely used as an antipyretic and analgesic. In some countries it is widely used for the treatment of rheumatism. In the gastrointestinal tract, aspirin is partially saponified to form salicylic and acetic acids. Part of it is absorbed unchanged. Acetylsalicylic acid, as an ester formed by acetic acid and phenolic acid (instead of alcohol), is very easily hydrolyzed. Already when standing in humid air, it hydrolyzes into acetic and salicylic acids. In this regard, pharmacists often have to check whether acetylsalicylic acid has been hydrolyzed. For this purpose, the reaction with FeCl3 is very convenient: acetylsalicylic acid does not give color with FeCl3, while salicylic acid, formed as a result of hydrolysis, gives a violet color.
Methyl salicylate-- salicylic acid methyl ester - is a liquid that is well absorbed by the skin. It is used as an external rub for the treatment of rheumatic and neuralgic pain, often in combination with other remedies. Methyl salicylate has both local and resorptive effects. (Fig. 3)
Salicylamide(like salicylic acid) sublimes when heated. Salicylamide is slightly soluble, osalmide is practically insoluble in water. Salicylamide is soluble in ethanol, moderately soluble in ether, slightly soluble in chloroform. Osalmid is easily soluble in ethanol and alkali solutions, moderately soluble in ether. (Fig. 4)
Phenyl salicylate, or salol, first obtained by our compatriot M.V. Nenetsky. Phenyl salicylate is a crystalline powder, very poorly soluble in water. Has free phenolic hydroxyl. Due to its low solubility in water in aqueous solutions, it does not give a color reaction with FeCl3, but its alcoholic solutions are colored purple by FeCl3. Phenyl salicylate hydrolyzes slowly. In medicine, it is used as a disinfectant for certain intestinal diseases. Its action is associated with hydrolysis and the release of salicylic acid and phenol. Phenyl salicylate is used to coat pills in cases where they want medicinal substances to pass unchanged through the stomach and exert their effect in the intestines: phenyl salicylate, generally hydrolyzing slowly, is only hydrolyzed to a very small extent in the acidic contents of the stomach and therefore the pill coatings from it disintegrate sufficiently only in the intestines.
Of the other salicylic acid derivatives, p-aminosalicylic acid (PAS) is of greater importance. It is synthesized by carboxylation, like salicylic acid. The starting compound in this case is m-aminophenol.
PAS has an antituberculosis effect and is used in the form of a sodium salt. Other isomers of this acid do not have such effects, and m-aminosalicylic acid, on the contrary, is a highly toxic substance. The proto-tuberculosis effect of PAS is explained by the fact that it is an antagonist of p-aminobenzoic acid, which is necessary for the normal functioning of microorganisms.
Preparations Sak
salicylate analgesic antipyretic
Phenacetin(Phenacetinum), FVIII. White crystalline powder or scaly crystals, odorless, slightly bitter in taste, almost insoluble in water. It is used orally in powders or tablets of 0.25-0.5 g per dose 1-3 times a day, depending on the indications. Often combined with other antipyretics or sedatives, as well as caffeine.
Antipyrine(Antipyrmum), FVIII (B). White crystalline powder, odorless, slightly bitter taste, highly soluble in water. It is used orally in powders or tablets of 0.25-0.5 g 1-3 times a day. Often used in combination with other agents. To stop bleeding, it is used externally in 10-20% solutions.
Higher doses: 1 g (3 g).
Pyramidon(Pyramidonum), FVIII (B). White crystalline powder, odorless, bitter taste, soluble in water. It is used orally in powders and tablets of 0.25--0.5 g 1--3 times a day. Often combined with other drugs. Its combination with veronal (1 mol: 2 mol) is called verodona.
Analgin(Analginum), FVIII (B). White crystalline powder, odorless and tasteless, highly soluble in water. Analgin solutions are unstable during storage. It is used orally in powders or tablets of 0.3-0.5 g and parenterally (subcutaneously, intramuscularly or intravenously) 0.5 g 1-3 times a day.
Higher doses: 1 g (3 g).
Butadion(Butadionum) (B). White crystalline powder with a weak aromatic odor and slightly bitter taste, almost insoluble in water, soluble in alkalis. It is used orally in powders or tablets of 0.15 g 4 times a day during the main course of treatment. Maintenance doses are 0.1-0.2 g per day. Butadione sodium salt can be used for intramuscular injections, although they are somewhat painful. A solution containing sodium salt of butadione and pyramidone in equal quantities is convenient for injection.
Sodium salicylate(Natrium salicylicum), FVIII. White crystalline powder or flakes, odorless, sweetish-salty taste, highly soluble in water. The drug is taken orally in powders, tablets or solutions, and is also administered intravenously in 10-15% solutions. A single dose of sodium salicylate is 0.5-1 g, the daily dose in the initial period of treatment of rheumatism can be 8-10 g. Subsequently, the dose is reduced. The total duration of treatment varies.
Biological effect of salicylates
Salicylates are non-steroidal anti-inflammatory drugs that have antipyretic (antipyretic), analgesic, and anti-inflammatory effects, and aspirin also has antiplatelet (reduces platelet aggregation) and anti-gout effects.
The main mechanism of action of salicylates as pharmacological drugs is the irreversible inactivation by acetylation of both isoforms of COX, a key enzyme in the synthesis of prostaglandins, prostacyclins and thromboxane from arachidonic acid.
It is assumed that the anti-inflammatory effect of AA and other salicylates is not limited to the effect on the prostaglandin system. Thus, acetylated COX-2 can form 15-R-hydroxyeicosatetraenoic acid, which is converted by 5 lipoxygenase into 15-epilipoxin A4, which has a powerful anti-inflammatory effect and enhances the effect of salicylates. In addition, salicylates reduce the activity of hyaluronidase and limit the energy supply of the inflammatory process by inhibiting the formation of ATP.
It is known that salicylates in large doses inhibit the contraction of striated muscles, and AK inhibits the spasmogenic effect of prostaglandins on smooth muscles.
The negative effect of salicylates on the body is associated with their inhibitory effect on the isoform of the COX enzyme - COX-2. Such side effects include ulcerogenic effects (the appearance of stomach ulcers and gastric bleeding), drug-induced liver damage (a rare complication in the form of hepatitis or liver failure), Reye's syndrome.
The ulcerogenic effect of aspirin is due to inhibition of blood coagulation factors and inhibition of the synthesis of prostaglandin E1, which has a cytoprotective effect on the gastric mucosa, and CaA formed during its breakdown inhibits the intestinal microflora.
Reye's syndrome is an acute encephalopathy in combination with fatty degeneration of the liver and other internal organs, which occurs after taking AK or other salicylates for viral infections (influenza, chicken pox, hepatitis A, AIDS), without treatment it ends in death. This disease affects children aged 4 to 16 years. The pathogenesis of Reye's syndrome is associated with mitochondrial damage that occurs under the influence of salicylates and viral infection.
In view of the side effects of salicylates described above, the creation of new dosage forms and products based on CaA and aspirin, devoid of their negative effects, is an important area of modern pharmacology. Some authors indicate that CaA derivatives with transition valence metals may have a number of beneficial pharmacological properties. Moreover, they do not cause side effects that are characteristic of SaK, and a number of other studies have noted that the anti-inflammatory effect of cobalt, zinc and copper salicylates is significantly higher than the similar effect exerted by SaK.
Posted on Allbest.ru
...Similar documents
Benzoic and folic acid and their derivatives. Para-aminobenzoic acid, its physicochemical properties. Biological effect and minimum daily intake of vitamin B10. Drug interactions. Anticonvulsants. Action of salicylates.
course work, added 04/13/2014
Classification of a group of drugs: pharmacokinetics, mechanism of action and pharmacodynamics, side effects, release forms and doses, pharmacotherapeutic features of drugs: acetylsalicylic acid (aspirin), ciprofloxacin, formoterol.
test, added 12/22/2015
Physicochemical properties of local anesthetics. Classification of drugs by chemical structure: esters and amides. Clinical and pharmacological characteristics of the drugs lidocaine, mepivacaine and articaine. Types of pain relief and systemic complications.
presentation, added 12/21/2015
Causes of development of atherosclerosis and coronary heart disease. Main components of lipids. Classification of hyperlipidemias. Determination of triglyceride levels. Lipid-lowering drugs. Bile acid sequestrants, statins, nicotinic acid, fibrates.
presentation, added 02/05/2015
Anticholinesterase agents with reversible mediator action, indications for the use of atropine. Medicines, indications and contraindications for their use. Group analogues of drugs, their pharmacological action and side effects.
test, added 01/10/2011
Erythropoiesis stimulants: epoetins, cyanocobalamin, folic acid, iron supplements. Drugs that stimulate and inhibit leukopoiesis. Medicines that affect blood clotting and blood clotting. Drugs to stop bleeding.
abstract, added 04/23/2012
History of the creation of antiviral drugs and their classification: interferon, interferon inducers, derivatives of amantadine and other groups of synthetic compounds, nucleosides. Antiviral drugs of plant origin. Receiving medications.
course work, added 01/31/2008
General characteristics, properties and methods of preparation, general methods of analysis and classification of alkaloid preparations. Phenanthrene isoquinoline derivatives: morphine, codeine and their preparations, obtained as semi-synthetic ethylmorphine hydrochloride; sources of receipt.
course work, added 02/13/2010
Classification of anti-tuberculosis drugs by the International Union Against Tuberculosis. Combination of isoniazid and rifampicin. Isonicotinic acid hydrazide preparations. Combined anti-tuberculosis drugs, their drug interactions.
presentation, added 10/21/2013
General characteristics of sedatives, their classification and mechanism of action. Main indications for use, side effects and contraindications. Benzodiazepine derivatives, drugs with antineurotic effects, a group of combined drugs.
Salicylic acid
Chemical formula of the product: C 7 H 6 O 3 / HOC 6 H 4 COOH
Product trade names:
O-Hydroxybenzoic acid
Phenol-2-carboxylic acid
Salonil
2-Hydroxybenzoic acid
2-Hydroxybenzenecarboxylic acid
2-Carboxyphenol
O-Carboxyphenol
Product Description:
Salicylic acid - white crystalline powder or needle-shaped crystals with a sweetish taste; Soluble in acetone, ether, alcohol, boiling water, benzene and turpentine, rarely soluble in chloroformbenzene, slightly soluble in water; Melts at 158°C. Form sodium salt (sodium salicylate) is a common one, obtained mainly from sodium phenolate with carbon dioxide under heat and pressure. Salicylic acid contains both a hydroxyl and a carboxyl group, which react with either acid or alcohol. The carboxyl group forms esters with alcohols; For example, methyl salicylate is formed with methanol, which is used in food flavorings and preservatives; Menthyl salicylate is formed with methanol, which is used in tanning lotions. The hydroxyl group reacts with acetic acid to form acetylsalicylic acid(so-called aspirin), which is the most common antiseptic and antipyretic agent. Phenyl salicylate (called salol) is formed by phenol, which is also used as an antiseptic and antipyretic. Sodium salt (sodium salicylate), a shiny white powder, used for antiseptic preparations and as a preservative. In addition to its analgesic and antipyretic properties, salicylic acid has keratinolytic properties and fungicidal properties. It and its derivatives are used in the treatment of hyperkeratosis, dandruff, ichthyosis and psoriasis, as well as in the treatment of fungal skin infections such as herpes zoster. Para-aminosalicylic acid (abbreviated PAS and PASA) is an analogue of para-aminobenzoic acid (abbreviated PABA), which inhibits the synthesis of folic acid in Mycobacterium tuberculosis and is bacteriostatic, inhibiting the growth and reproduction of tubercle bacilli. Para-aminosalicylic acid and its sodium salt (sodium p-aminosalicylate) are bacteriostatic against mycobacteria and are used to treat tuberculosis; Orally. Aminosalicylic acids are pharmaceutically active ingredients, including anti-infectives against colds, flu and other viral infections. Mesalamine (5-aminosalicylic acid, abbreviated 5-ASA) is an active metabolite of sulfasalazine used to treat inflammation of the rectum and lower colon, proctosigmoiditis, mild to moderate ulcerative colitis, and proctitis. Para-aminosalicylic acid (4-hydroxybenzoic acid) is used as a bacteriostatic intermediate, especially for arabens (alkyl esters of p-hydroxybenzoic acid), which are used in food and personal care products as a preservative. It is used in the production of liquid crystal polymers. It is also used as an intermediate in dyes, insecticides, pharmaceuticals, pesticides and other chemical compounds. Salicylic acid and its derivatives are important for the preparation of other pharmaceutical products, dyes, flavors and preservatives. Topical keratolytic agents are beta hydroxy acids such as salicylic acid.
If you have heard about salicylic acid, chances are you know it as the main ingredient in aspirin. The chemical got its name from the Latin term for willow, salix, because it was first made from a complex carbohydrate found in willow bark. There are some companies that make acne care products claiming to contain salicylic acid from willow bark, but the compound is not found in tree bark. Powdered bark must be treated with oxidizing agents and filtered to obtain acid. Salicylic acid is a very useful pain reliever. For a while, researchers even speculated that it might be a vitamin, which they called vitamin C. Inside the Body salicylic acid relieves pain and improves blood circulation. When applied to the skin, it breaks down oily compounds such as oily sebum that can clog pores. In fact, it is so good at breaking down fats and oil-like compounds in the skin that it is generally believed that more than 2% is used on the face. salicylic acid, with 98% of the lotion being a neutral carrier. Until 3% salicylic acid can be used on other parts of the body, and 10% to 30% will dissolve warts. Using a soft solution salicylic acid directly on the skin offers many of the benefits of cleansing, without the risk of tearing pores or damaging tiny blood vessels. However, treatment salicylic acid has many benefits that a simple cleaning procedure does not. Gently removing dead skin does more than just open pores. Salicylic acid increases cell turnover. This causes the skin to grow faster, opening up the pores. It increases collagen production, filling cavities in the skin and making it less “flexible.” It removes discoloration from skin, although it is often too strong to use on dark skin. Salicylic acid is the only beta hydroxy acid used in skin care. It performs the same skin care tasks as alpha hydroxy acids such as lactic acid and glycolic acid, but is used in a much weaker concentration. Acne care products can contain up to 30% alpha hydroxy acids, but the same effect is achieved with 0.5% to 2% salicylic acid. Similar to benzoyl peroxide salicylic acid It is most effective only when used continuously even after the acne has cleared. In the absence of exfoliating and cleansing action salicylic acid pores can become clogged again, causing acne to return. Salicylic acid also used in many acne treatments as combination therapy at low concentrations. The exfoliating effect of the acid helps enhance the effectiveness of other active ingredients. Because salicylic acid is effective at low concentrations, it is significantly less irritating than other products.
Chemical peeling is a safe, effective and economical procedure for treating various skin diseases and improving appearance. Principle peeling involves controlled chemical damage to the skin to induce skin rejuvenation, resulting in smoother skin and improved surface texture. Chemical peeling can be classified in different ways. A useful approach is to classify them according to the degree of skin damage, which determines the indications they can be used for treatment. Respectively, chemical peeling can be divided into three broad categories, i.e. superficial, mid-depth and deep. Superficial peelings cause damage to the epidermis and are therefore used to treat superficial conditions, including melasma, acne and dyschromia. Medium depth peels penetrate the papillary dermis and are useful in the treatment of solar keratoses, dyschromia and pigment disorders. Deep peels cause necrosis down to the level of the reticular dermis, so they are indicated for deep wrinkles, severe photoaging and deep scars. Salicylic acid is part of a group of compounds known as hydroxy acids, which are widely used for a number of cosmetic indications due to their many important properties. Its mechanism of action is desmolytic rather than a true keratolytic and is safe in dark-skinned individuals. Chemical peeling is the process of causing controlled chemical damage to the skin (partial or complete epidermis with or without dermis) by applying a chemical peel that causes the superficial layers of the skin to peel off, resulting in the removal of superficial lesions followed by the regeneration of new epidermal and dermal tissues. Salicylic acid is generally a safe compound when used in appropriate concentrations for the treatment of acne. However, one thing you may notice with salicylic acid-based acne products is that they can sometimes leave your skin a little dry. So, it goes without saying that you should avoid any harsh cleansers or astringents when using salicylic acid products. It's important to make sure you have a balanced acne treatment regimen, especially if you're using salicylic acid-based products. Make sure you moisturize your skin regularly and use soothing products when using salicylic acid. Also, make sure you don't apply salicylic acid For large areas of your skin, stick to areas that have acne. If your skin is damaged, swollen, red, or infected, avoid using salicylic acid-based products.
Physico-chemical properties Salicylic acid.
|
Indicators |
Meaning |
|
|
State of aggregation Salicylic acid |
crystalline powder |
|
|
Color Salicylic acid |
White to pale yellow |
|
|
Melting point Salicylic acid |
158-161°C |
|
|
Boiling point Salicylic acid |
211°C |
|
|
Density Salicylic acid |
1,44 |
|
|
Vapor Density Salicylic acid |
||
|
Vapor pressure Salicylic acid |
1 mmHg Art. (114°C) |
|
|
Solubility: ethanol: 1 M at 20°C |
transparent, colorless |
|
|
Solubility in water |
1.8 g/l (20 °C) |
|
|
pH level Salicylic acid |
Storage and transportation Salicylic acid.
Salicylic acid has the ability to destroy lipids in the skin, causing symptoms ranging from dryness and irritation at low concentrations to mild acid burns at higher concentrations. If ingested in large quantities, it can cause salicylate toxicity, which can lead to very serious side effects.
No special storage is required; salicylic acid can be stored in any clean container. Keep closed. Keep away from heat. Keep away from sources of ignition. Empty containers pose a fire hazard; evaporate residues using a hood. Ground all equipment containing the material. Do not swallow. Do not inhale dust. Wear appropriate protective clothing. In case of insufficient ventilation, wear suitable respiratory equipment. If swallowed, seek immediate medical attention and show container or label. Avoid contact with skin and eyes. Keep away from incompatible substances such as oxidizing agents, moisture.
Product Application Areas .
Salicic acid used as an anti-aging agent
Salicic acid It is used as a remedy for poisoning by certain poisons.
Salicic acid used as a means to remove warts and other skin defects.
Salicic acid used as a cosmetic biocide.
Salicic acid used as denatured alcohol.
Salicic acid used as an exfoliant.
Salicic acid used as an external analgesic.
Salicic acid used as a flavoring agent.
Salicic acid Used as a skin conditioner.
Salicic acid used as a preservative.
Salicic acid used as an agent in hair conditioning products.
Salicic acid used as a solvent.
Salicic acid used as an agent in sun protection creams.
Salicic acid used in the production of ultraviolet light absorption products.